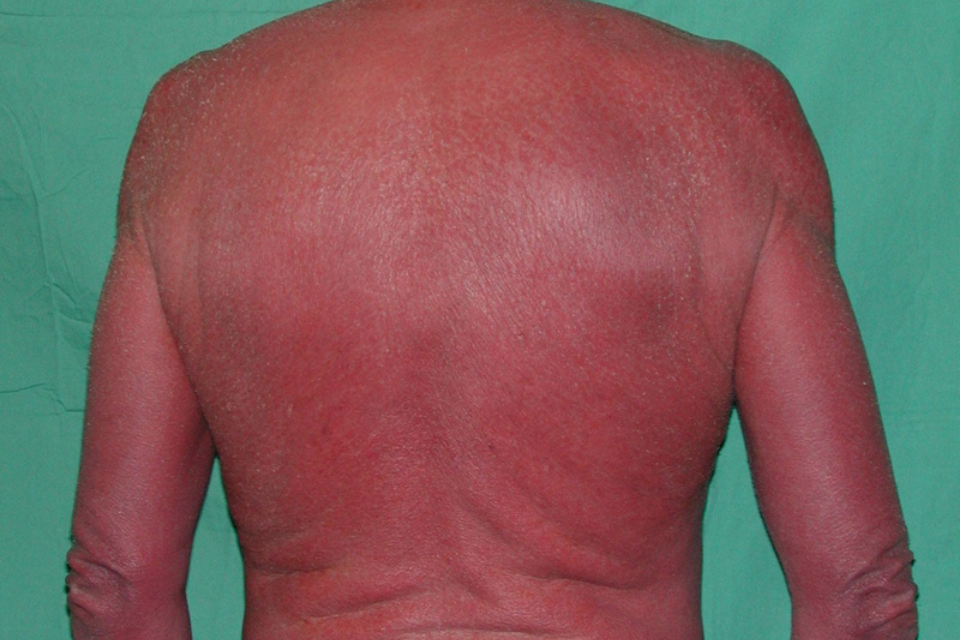
 Erythroderma (exfoliative dermatitis) is a clinical syndrome characterized by extensive erythema involving greater than 90 percent of the skin surface. It may be the result of many different dermatological conditions including preexisting dermatoses, drug reactions and malignancy. Hospitalization may be required for initial evaluation and treatment since many of these patients are elderly and the skin involvement is extensive leading to significant mortality risk. Patients need to be monitored to ensure that temperature, water, protein and electrolyte homeostasis are maintained. Erythroderma accounts for 1% of all dermatologic hospital admissions.
Erythroderma (exfoliative dermatitis) is a clinical syndrome characterized by extensive erythema involving greater than 90 percent of the skin surface. It may be the result of many different dermatological conditions including preexisting dermatoses, drug reactions and malignancy. Hospitalization may be required for initial evaluation and treatment since many of these patients are elderly and the skin involvement is extensive leading to significant mortality risk. Patients need to be monitored to ensure that temperature, water, protein and electrolyte homeostasis are maintained. Erythroderma accounts for 1% of all dermatologic hospital admissions.
Epidemiology:
Erythroderma is a rare skin disorder with an estimated annual incidence of 1 to 2 patients per 100,000 population. It is more common in males with a male to female ratio of 2:1 to 4:1. Erythroderma occurs more commonly in older adults, with affected patients usually over 45 years of age and a mean age of onset of 55 years, although it should be noted that most studies exclude pediatric cases of erythroderma. Approximately 50% of cases are exacerbations of preexisting dermatoses such as psoriasis, atopic dermatitis, or pityriasis rubra pilaris. Other causative factors include drug reactions (20 percent), malignancies (11 percent), and idiopathic (17 percent).
Etiology:
A wide range of cutaneous and systemic diseases can cause erythroderma. It is important to establish the correct diagnosis whenever possible because specific therapy other than corticosteroids or anti-inflammatory treatments may be necessary to improve the patient’s condition.
Dermatoses:
Erythroderma occurring secondary to a preexisting skin condition is the most common etiology. The underlying skin condition is commonly known prior to onset of the erythroderma; however, some may initially present with erythroderma. In analyzing the combined data from the studies listed in table 1, psoriasis was the most common (23.1 percent) followed by atopic dermatitis/eczema (9.5 percent), pityriasis rubra pilaris (3.4 percent), contact dermatitis (3.2 percent), actinic dermatitis (2.7 percent) and seborrheric dermatitis (2.5 percent). Additionally, pemphigus foliaceus, lichen planus, icthyosiform dermatitis, phytophotodermatitis, stasis dermatitis, extensive dermatophytosis, Norwegian scabies, senile xerosis, and staphylococcal scalded skin syndrome account for the remaining cases. Table 7.2 lists the inflammatory dermatoses associated with erythroderma. It is important to consider other possible etiologies even in patients with clear preexisting dermatoses. Eugster et al described that 5 out of 7 patients with malignancy-induced erythroderma had a history of a preexisting dermatosis.
Psoriasis:
Erythrodermic psoriasis is a rare and severe form of psoriasis occurring in 1-2.25% of patients with psoriasis. It typically occurs in patients with established psoriasis with an average of 14 years between the onset of psoriasis and the first erythrodermic episode although in some cases, it can be the patient’s initial presentation of psoriasis. Erythrodermic psoriasis may present acutely or may run a chronic course with frequent relapses. There are a variety of medical and social conditions that may trigger outbreaks of erythroderma in previously localized psoriasis including systemic illness such as HIV infection, discontinuation of medications (including oral corticosteroids, potent topical steroids, methotrexate or biologics), emotional stress, UV burn, topical tar, and alcoholism. The classic plaques of psoriasis may be present in the early and remitting stages of erythroderma. The patient may also have the classical nail changes and arthritis associated with psoriasis.
Pityriasis Rubra Pilaris
Pityriasis rubra pilaris (PRP) may initially resemble a seborrheic dermatitis-like eruption of the scalp that then progresses into erythroderma over weeks to years. A similar picture of eruption on the scalp, with additional involvement of butterfly regions of the face and upper trunk can also be seen in early erythrodermic pemphigus foliaceus. The erythroderma in PRP classically appears as generalized salmon-colored erythema with islands of sparing. Islands of sparing may also be seen in psoriasis, cutaneous T-cell lymphoma and sarcoidosis. Patients with PRP may also have underlying follicular papules and keratoderma of palms and soles.
Drug Hypersensitivity Reactions:
Both topical and systemic drugs are notorious for precipitating erythroderma, accounting for 20 percent of cases. While this list is constantly expanding, many of the implicated drugs come from single case reports. The most commonly implicated drugs include allopurinol, arsenicals, aspirin, carbamazepine, captopril, gold, hydantoins, mercurials, penicillin, phenothiazines, phenylbutazone, quinacrine, and sulfonamides. Additionally homeopathic, unani, ayurvedic, herbal, and home remedies have also been reported to cause erythroderma. Interestingly, while carbamazepine is known to induce erythroderma, Smith et al. reported a case where the carbamazepine successfully cleared erythrodermic psoriasis.
Drug-induced erythroderma typically occurs rapid and resolves quickly – on average two to six weeks after discontinuation of the drug. Drug eruptions that initially began as morbilliform, lichen planus-like or urticarial may evolve into erythroderma. Nicolis et al. reported that erythroderma caused by sulfonamides, penicillins, antimalarials, barbiturates, arsenicals and mercurials tended to have a more severe clinical course. Fever appears to be prominent in these cases, and moderate to severe liver degeneration can occur. Drug-induced erythroderma due to dapsone or antileprosy agents can mimic the clinical and histolopathologic features of cutaneous T-cell lymphoma. In this situation, the erythroderma resolves with withdrawal of offending drugs.
Malignancy:
Erythroderma may be a clinical expression of both hematological and internal malignancies, accounting for approximately 11% of cases. Cutaneous T-cell lymphoma is the most common etiology, accounting for 74 percent of the malignancy-induced erythroderma and 7.8 percent of all cases of erythroderma. Other malignancies include Hodgkin’s lymphoma (11 percent), internal malignancy (8.5 percent), leukemia (4.2 percent) and non-CTCL, non-Hodgkin’s lymphoma (2.1 percent). Table 4 lists the specific hematologic, and internal malignancies implicated in erythroderma. Malignancy should be considered in the differential diagnosis for erythroderma, especially when the disease is insidious, debilitating, or progressive in course, when the patient has no history of preexisting dermatoses, or when the erythroderma is refractory to treatment.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphoma (CTCL), also known as mycosis fungoides, is the most frequently identified malignancy to cause erythroderma, and Sézary syndrome is the most common presentation associated with erythroderma. Sézary syndrome is characterized by peripheral blood involvement of Sézary cells. The Sézary cell is an atypical lymphocyte with the characteristic morphology of a cerebriform nucleus and ample cytoplasm with vacuoles. It is controversial how many Sézary cells are needed to define Sézary syndrome. The original NCI classification used the criterion of greater than 5%, but current practice at mycosis fungoides referral centers prefer greater than 20% or an absolute count of at least 100/mm3 Sézary cells. In both Sézary syndrome and non-Sézary mycosis fungoides, erythroderma may be accompanied by poikiloderma or frank tumors. Patients also have intense pruritus which may be so severe as to interfere with daily activities. The diagnosis should be considered in all cases of erythroderma when the patient has infiltration of the skin, marked or asymmetric lymphadenopathy or splenomegaly. In severe cases of Sézary syndrome, patients may have leonine facies and hyperkeratosis of palms and soles with painful fissuring. Erythroderma may be the first sign of CTCL, and its clinical and histological appearance may be identical to that of benign erythroderma. In these cases, flow cytometry immunophenotyping of lymphocytes and T-cell receptor gene analysis are useful in identifying CTCL. Sézary syndrome has a poorer prognosis than mycosis fungoides with a median survival of 2.5 to 5 years.
Internal Malignancy
Internal malignancies are implicated in 1 percent of all cases of non-CTCL related erythroderma. Frequently, the malignancy is occult at the initial presentation of erythroderma. The common types of causative malignancies cited in the literature include carcinoma of the lung, prostate, thyroid, rectum, stomach, and liver.
Miscellaneous Diseases
Erythroderma is also associated with several disorders that do not fit into the aforementioned groups. These disorders include graft-versus-host disease (GVHD), hepatitis, irradiation, reactive arthritis (formerly know as Reiter’s syndrome), sarcoidosis, acquired immunodeficiency, congenital immunodeficiency syndrome and disseminated histoplasmosis. Japanese patients have been reported to develop a potentially fatal postoperative erythroderma caused by a unique form of transfusion-related GVHD and this can be prevented by the use of irradiated blood for transfusions. The clinical findings in erythroderma caused by reactive arthritis may mimic the appearance of erythrodermic pustular psoriasis. Important distinguishing features of reactive arthritis include recent history of gastroenteritis, cervicitis in women or urethritis in men, mouth ulcers, or iridocyclitis. The erythroderma that can occur secondary to progressive disseminated Histoplasma capsulatum infection can closely resemble CTCL. The diagnosis of Histoplasma should be suspected when chronic ulcers with an indurated base occur on the skin or oral mucosa or if characteristic chorioretinal lesions occur in the eyes.
Pathogenesis:
One intriguing aspect of erythroderma is the fact that clinically distinct skin diseases can progress into erythroderma with a relatively uniform clinical picture. The pathogenesis of erythroderma is not completely understood and it is still unclear whether the underlying mechanisms leading to erythroderma are the same or different depending on the underlying disease. The development of erythroderma is thought to be secondary to a complex interaction of cytokines and cellular adhesion molecules including interleukins (IL)-1, -2, -4, -5 and -10, tumor necrosis factor (TNF), interferon (IFN)- (gamma), intercellular adhesion molecule-I (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), selectin-E and –P. Siggurdsson et al found that while all types of erythroderma have a predominately CD4+ T-cell lymphocytic infiltrate in the skin, Sézary syndrome tends to have CD4+ T-cells that secrete IL-4, consistent with the Th2 subset of T-helper (Th) cells while benign forms of erythroderma such as atopic dermatitis and psoriasis have a cytokine profile characterized by IFN- (gamma) secretion, consistent with the Th1 subset. Siggurdsson et al also found that atopic dermatitis-induced erythroderma, CTCL-induced erythroderma and idiopathic erythroderma all had increased levels of circulating adhesion molecules, and the types of adhesion molecules expressed were not significant between the different causes.
These molecular, cellular, and immunologic changes lead to increased mitotic rate and increased number of germ skin cells, resulting in a dramatic increase in the epidermal turnover rate. This increased turnover rate leads to greater loss of viable cellular material such as nucleic acids, amino acids, protein and folic acid. Erythroderma may increase daily protein loss through loss of keratinocytes and scaling by 25-30 percent in psoriatic erythroderma and by 10-15 percent in other causes of erythroderma.
Clinical Manifestations:
Erythroderma is defined as generalized erythema and scaling of at least 90 percent of the patient’s skin surface. The condition typically begins as patches of erythema accompanied by pruritus. These patches then enlarge and coalesce, eventually resulting in involvement of most of, or in some cases all of, the skin surface area. The palms, soles, and mucous membranes are generally spared however, erythroderma that is secondary to cutaneous T-cell lymphoma or pityriasis rubra pilaris may involve the palms and soles. The character of erythema varies with the duration of the disorder with sudden-onset lesions typically bright red and more chronic lesions generally deeper, dusky red. In darker-skinned patients, the erythema is darker from the onset. The degree of scaling can vary greatly between cases from virtually none to extensive. The scaling usually begins within several days of the onset of erythema, classically beginning in the flexures. The size of the scale also varies with the duration of symptoms, with larger scale in acute cases and smaller scale in chronic. Secondary bacterial infections are a frequent complication and can cause crusting, impetigo, or folliculitis.
Nail examination in erythrodermic patients frequently reveals dystrophy, especially in patients whose erythroderma is secondary to psoriasis. The nail plate is often thickened and lusterless with horizontal depressions (Beau’s lines). Subungual hyperkeratosis, the accumulation of keratinaceous material under the nail plate, may occur leading to distal onchycholysis, separation of the nail plate from the nail bed. The entire nail plate may be shed in severe cases. Additionally, the patient’s hair may also be affected. Alopecia occurs in 25 percent of patients and more often develops in patients with severe forms of exfoliation.
Most patients complain of pruritus, which may be mild to severe. Some patients also experience pain or a burning sensation of the skin. Constitutional symptoms such as fever, chills and malaise are common. Both hyperthermia and hypothermia may be observed. Heat loss is a major concern secondary to the defective skin barrier. There is loss of normal vasoconstrictive function of the dermis, decreased sensitivity of the shivering reflex and increased heat loss from evaporation of weeping fluids from the skin. These processes can lead to increased basal metabolic rate and a catabolic state resulting in significant weight loss. Patients may develop either regional or generalized lymphadenopathy. Axillary and inguinal lymphodenopathy is more common than cervical lymphodenopathy. Generalized lymphadenopathy is more common in patients whose erythroderma is secondary to malignancy. Mild hepatomegaly may occur in up to 20 percent of cases regardless of etiology; however, splenomegaly is usually only seen in patients with underlying malignancy, sarcoidosis, histoplasmosis or severe hypersensitivity reactions. Other nonspecific clinical findings include peripheral edema, gynecomastia and high output cardiac failure, which results from peripheral vasodilation.
Laboratory Findings:
The laboratory findings are often not helpful in uncovering the underlying cause of erythroderma. Common abnormalities include mild anemia, leukocytosis, elevated sedimentation rate, hyperuricemia, hyperglobulinemia, hypoalbuminemia, and eosinophilia. Mild anemia, with hematocrit values between 35 and 38 percent, is thought to be due to folic deficiency, iron deficiency, and a chronic inflammatory state. More severe anemia is unusual. Leukocytosis of greater than 20,000 leukocytes/mm3 is seen in both benign and malignant forms of erythroderma. Eosinophilia and increased IgE are frequently observed findings in erythroderma secondary to atopic dermatitis and drug reactions but are not diagnostic for these conditions. Griffiths et al. observed transient CD4+ T-cell lymphocytopenia in HIV negative patients with acute erythroderma as a consequence of T-cell sequestration in the skin.
For the diagnosis of Sézary syndrome, Sézary cell count analysis can be useful. In current practice, greater than 20 percent or absolute count of at least 100/mm3 circulating Sézary cells is diagnostic for Sézary syndrome. Less than 10 percent circulating Sézary cells is nonspecific and is seen in a variety of benign dermatoses including contact dermatitis, atopic dermatitis, psoriasis, lichen planus, discoid lupus, and parapsoriasis. Differentiating Sézary syndrome from erythrodermic actinic reticuloid can be difficult since the two diseases can have similar clinical presentations and high levels of circulating Sézary cells in the peripheral blood. However, immunophenotyping and nuclear contour index (NCI) of blood lymphocytes can help differentiate the two since actinic reticuloid has increased CD8+ T-cells and decreased NCI compared to Sézary syndrome. Additionally, actinic reticuloid patients are photosensitive. Looking for clonal T-cell populations in peripheral blood smears through T-cell receptor gene analysis can help to distinguish CTCL from benign erythroderma. This technique can also be applied to skin biopsies.
Histopathology:
Skin biopsy is an important diagnostic tool for identifying the etiology of the erythroderma, but interpretations of these specimens may be difficult. This difficulty is due to the fact that the histopathological expression of the underlying disorder tends to be more subtle in the setting of erythroderma than in the conventional disease form. Multiple skin biopsies submitted simultaneously can increase the accuracy of histopathological diagnosis, as the histopathological manifestations of the underlying disease are often heterogeneously distributed in the affected skin. Studies comparing “blinded” microscopic diagnosis with final diagnosis found a 31 to 66 percent correlation. Walsh et al found that the correct pathological diagnosis is reached more often in patients with dermatitis, CTCL and psoriasis than in patients with drug eruptions or PRP.
It is especially important to differentiate benign inflammatory erythroderma from Sézary syndrome given the aggressive course of this lymphoma. Ram-Wolff et al found that this distinction could be made with certainty in 57 percent of cases. Distinguishing histopathological features of Sézary syndrome include Pautrier microabcesses, atypical lymphocytes, basilar lymphocytes and dense dermal infiltrate. The basal lymphocytes seen in CTCL can be difficult to distinguish from the interface (dermal-epidermal) inflammation seen in drug eruptions. Identification of numerous dilated blood vessels favors a diagnosis of benign inflammatory erythroderma.
Treatment:
All cases of erythroderma are considered a dermatological emergency, and hospitalization should be considered if fluid and electrolyte imbalance or cardiovascular or respiratory comprise occur. Regardless of the underlying etiology, the initial therapy for an erythrodermic patient should focus on fluid, electrolyte and nutritional management as well as gentle skin care measures. If a drug reaction has not been ruled out, all nonessential medications should be discontinued if possible. Oatmeal baths and wet dressings to weeping or crusted sites followed by bland emollients and low-potency topical corticosteroids can help to reduce inflammation and pruritus. High potency corticosteroids should be avoided due to increased systemic absorption from the extensive surface area involvement and increased permeability of erythrodermic skin. Topical tacrolimus has also been shown to be systemically absorbed and blood levels should be monitored if used to avoid nephrotoxicity in an erythrodermic patient. Sedating oral antihistamines can enhance this effect and relieve anxiety. Patients require a warm and humidified environment to increase comfort, prevent hypothermia and increase moisture to the skin. Diuretics may be needed to treat edema, and systemic antibiotics may be required for secondary bacterial infections.
Patients who are refractory to topical therapy should receive systemic therapy directed at the underlying etiology. First line treatment for erythrodermic psoriasis includes cyclosporine, infliximab, acitretin and methotrexate with cyclosporine and infliximab being more rapidly acting agents. While systemic corticosteroids should be avoided in patients with suspected underlying psoriasis because of the potential to induce a rebound flare, they are helpful in treating drug-induced and atopic dermatitis-related erythroderma. Pityriasis rubra pilaris induced disease responds well to retinoids and methotrexate. The best treatment regimen for Sézary syndrome depends on a variety of patient factors including burden of disease, impact on quality of life and rate of disease progression. Systemic therapies such as extracorporeal photopheresis, interferon-〈 (alfa), bexarotene, low dose methotrexate and denileukin diftitox can be used alone, or combined with skin directed therapies such as phototherapy, topical nitrogen mustard and total skin electron beam radiation.
When the etiology of erythroderma remains unknown, patients can be treated with empiric therapy which includes systemic corticosteroids, methotrexate, cyclosporine, mycophenolate mofetil, and actretin. Until CTCL has been ruled out by up-to-date laboratory testing, immunosuppressive agents should be avoided.
Complications:
Patients with erythroderma can suffer from a variety of serious complications including secondary infection, dehydration and electrolyte abnormalities, edema and high output cardiac failure. Patients with erythroderma have increased skin colonization of Staphylococcus aureus when compared to healthy controls. This colonization may explain the high incidence of staphylococcal septicemia in patients with erythrodermic psoriasis and CTCL. The rapid turnover of the epidermis leads to increased daily protein loss of 25-30 percent in psoriatic erythroderma and 10-15 percent in other causes of erythroderma which can lead to a net negative nitrogen balance as seen by muscle wasting, hypoalbuminemia, and edema. Increased cardiac output has been demonstrated in patients with erythroderma secondary to dilation of cutaneous blood vessels in the diseased skin which can lead to heart failure especially in patients with underlying cardiac disease or the elderly.
Prognosis:
The prognosis of erythroderma depends on the underlying disease. Drug induced erythroderma often resolves quickly once the offending agent is removed while CTCL often has a chronic and refractory course. While older series report high mortality from erythroderma ranging from 5 to 64 percent, this mortality has been likely reduced due to advances in diagnosis and therapy.